Independent, International and Industry-Leading
Medix Biochemica is a market-leading in vitro diagnostics raw material supplier with Finnish roots and global branches. We produce and supply high-quality antibodies, antigens and other critical IVD raw materials to enable our IVD customers to manufacture diagnostic tests and supporting materials all around the world. Our expertise covers market segments from immunoassay, clinical chemistry and molecular diagnostics. Our capabilities include biospecimen accruals and contract manufacturing. With the most comprehensive raw material portfolio in the IVD world, and a team made up of the best minds in the business, we’re ready to shorten your time to market and build quality into every one of your tests. Across disciplines and disease areas, whatever you need, chances are we IVDo that.
Discover Who We Are
Through a series of strategic acquisitions, portfolio expansions and other activities, we are well on our way to becoming the first-choice partner for the IVD industry. Learn more about our history here.
Get to Grips With Our Products
With over 5000 products available, we can supply over 95% of the most common reagents for assay development, manufacturing and validation. Find our comprehensive product catalog from here.
Find Out What We IVDo
We’ve invested in technologies to supply the highest quality of monoclonal antibodies while also expanding portfolios and capabilities to become a global, market-leading supplier to the in vitro diagnostics (IVD) industry. We can offer you a customized contract manufacturing service that makes your antibody production effortless. The process includes evaluation and optimization through cell line adaptation, mycoplasma testing, production optimization and validation.
Medix Biochemica Group Video
We've Got Offices and Manufacturing Facilities All Around the World
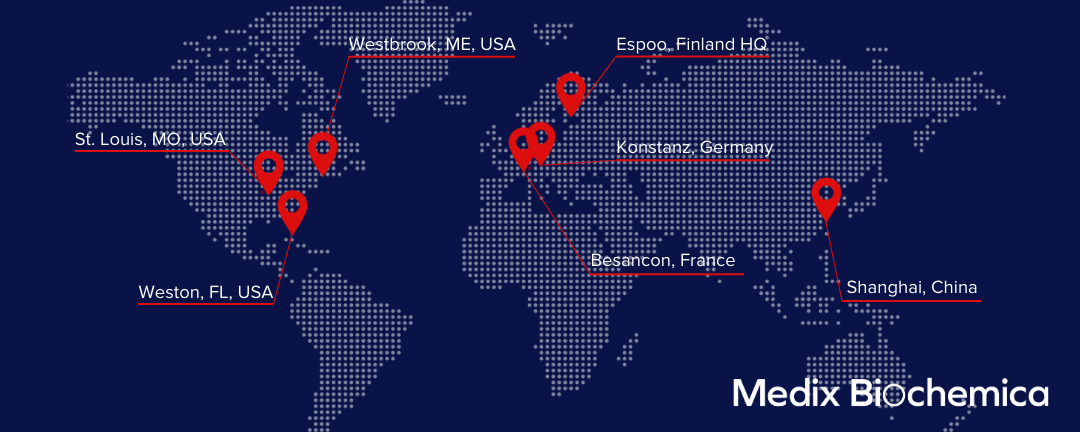
Medix Biochemica Across the World
Medix Biochemica is a global in vitro diagnostics (IVD) raw materials supplier with a portfolio of over 5 000 products, including antibodies, antigens, enzymes, biospecimens and molecular diagnostic reagents. Our expertise covers the immunoassay, clinical chemistry and molecular diagnostics markets, among others.
Medix Biochemica Acquires ViroStat
Medix Biochemica expands its infectious diseases antibody offering through the acquisition of ViroStat

.png)
